
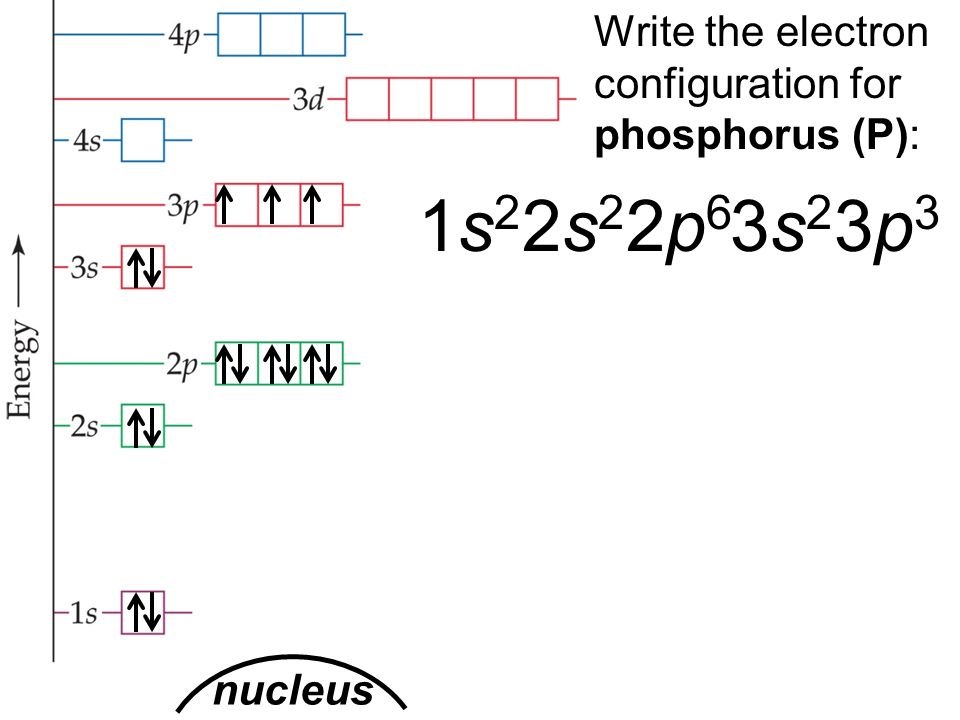
These orbitals contain a number of boxes that can hold a number of electrons. There are different types of orbitals – s, p, d, and, f.
#AS ELEMENT ORBITAL DIAGRAM FREE#
If you got any doubt, feel free to ask.If you understand the above rules then constructing the orbital diagram or orbital notation for any atom is super easy. I hope that now you can draw the orbital diagram of any element or ion. If there is one electron in an orbital, that orbital is called an unpaired orbital, and the electron is known as an unpaired electron. If there is a pair of electrons in an orbital, that orbital is called a paired orbital, and the two electrons are known as paired electrons. What are the paired and unpaired electrons? Paired electrons Then, let’s see what the paired electrons and unpaired electrons are. Thus, how different electron numbers are filled in the p orbital can be shown as follows. So, all the electrons in singly occupied orbitals have the same spin, and the two electrons in the same orbital or the electrons in doubly occupied orbitals have opposite spins.

Therefore, we can draw a pair of electrons in an atomic orbital as follows.Īccording to Hund’s rule, in an orbital, every subshell is singly occupied with one electron before any one orbital is doubly occupied. In other words, the two electrons in the same atom cannot have the same quantum numbers. The Pauli exclusion principle states that when there is a pair of electrons in an atomic orbital, the two electrons spin in opposite directions to each other. Here, the orbital which has the lowest energy is the same orbital nearest to the nucleus of the atom. The Auf Bau principleĪccording to the Auf Bau principle, electrons are filled to the energy levels around the nucleus from the orbital, which has the lowest energy to the orbitals in the order of increasing energy. They are the Auf Bau principle, the Pauli exclusion principle, and Hund’s rule. Here, three principles are important regarding the position of electrons in orbitals. Then you can draw arrows inside orbitals to represent electrons. Then you have to draw the orbitals according to the electron configuration of the element and name them accurately. When drawing orbital diagrams, you can use a circle or square to represent an orbital.
#AS ELEMENT ORBITAL DIAGRAM HOW TO#
Now, let’s talk about how to draw orbital diagrams. The differences between orbital diagram and electron configuration The direction of the arrowhead shows the spin of electrons in orbitals. Instead of numbers in electron configurations, there are arrows in orbital diagrams to indicate electrons. In simple words, orbital diagrams are pictorial representations of electron configurations. Here, I’m going to tell you about the orbital diagrams, what they are and how to draw orbital diagrams of atoms and ions, and also the differences between electron configurations and orbital diagrams. I promise there will be no doubt left about orbital diagrams after you go through this chapter. They show the distribution of electrons in each orbital of the atom in a way that anyone can easily understand. Do you wonder why? It’s because we can simply represent the electron configuration of an element by sketching an orbital diagram. Orbital diagrams are closely related to electron configurations. How to draw orbital diagrams step-by-step?


 0 kommentar(er)
0 kommentar(er)
